EMBO 2014 Team Clarity
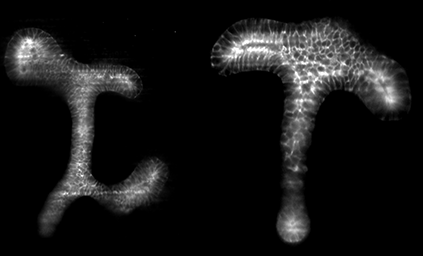
Our first try: 3D Reconstruction of Drosophila Embryo.
Mouse Development
Work plan during the course
- Samples of different sizes: 500(L)x300(R) um to 1(L)x0.7(R) cm including- Mouse embryos at different developmental stages (E10.5, E11.5, E12.5 & E13.5) & Brain, Lung & Heart of E18.5 embryo
- A variety of signal intensity (expression levels, stability, etc…) – Different “labeling” patterns: membrane GFP in all tissues in the context of an intact embryo or dissected embryonic organs, Cytoplasmic GFP in scattered cells at different regions of the embryo & Cytoplasmic GFP expressed exclusively in PGCs
- Samples of different morphology: opaque, transparent, branched, auto-fluorescent, etc…)
Imaging Results during the course:
Ultra microscope – Cleared E13.5 mouse embryo:
Branching organs
"Goal": 3D Life imaging of the thick, uncleared embryonic tissue with the light sheet microscopy.
Mouse embryonic kidneys at E11.5, E12 and E12.5 were imaged with Zeiss Lightsheet Z.1. Light sheet microscopy provides a cellular resolution at the organ scale. Green and red depict renal epithelium and mesenchyme.
Optical sectioning of embryonic mouse kidney with OpenSPIM. Greyscale depicts e-cadherin staining in the epithelium.
Take home message: commercial and home build light sheet microscopes can be used to image embryonic organ explants, however two photon and active optics might be necessary to fight scattering.
Investigation of 3d tissue growth using Light Sheet Microscopy
My aim was to use Light Sheet Microscopy to resolve the three-dimensional structure of fixed tissues grown in transparent straight sided pores of controlled shape. The transparent scaffolds have two different circular pore sizes and are fabricated with the aid of rapid-prototyping. Pre-osteoblast (MC3T3-E1) Cells were then seeded on top of the scaffolds and cultured in a cell culture media for up to 28 days. The grown tissue has been fixed at certain time-points and, in order to quantify the volumetric distribution of the tissue, the scaffolds were mounted in a sample holder for subsequent visualization with a Light Sheet Microscope.
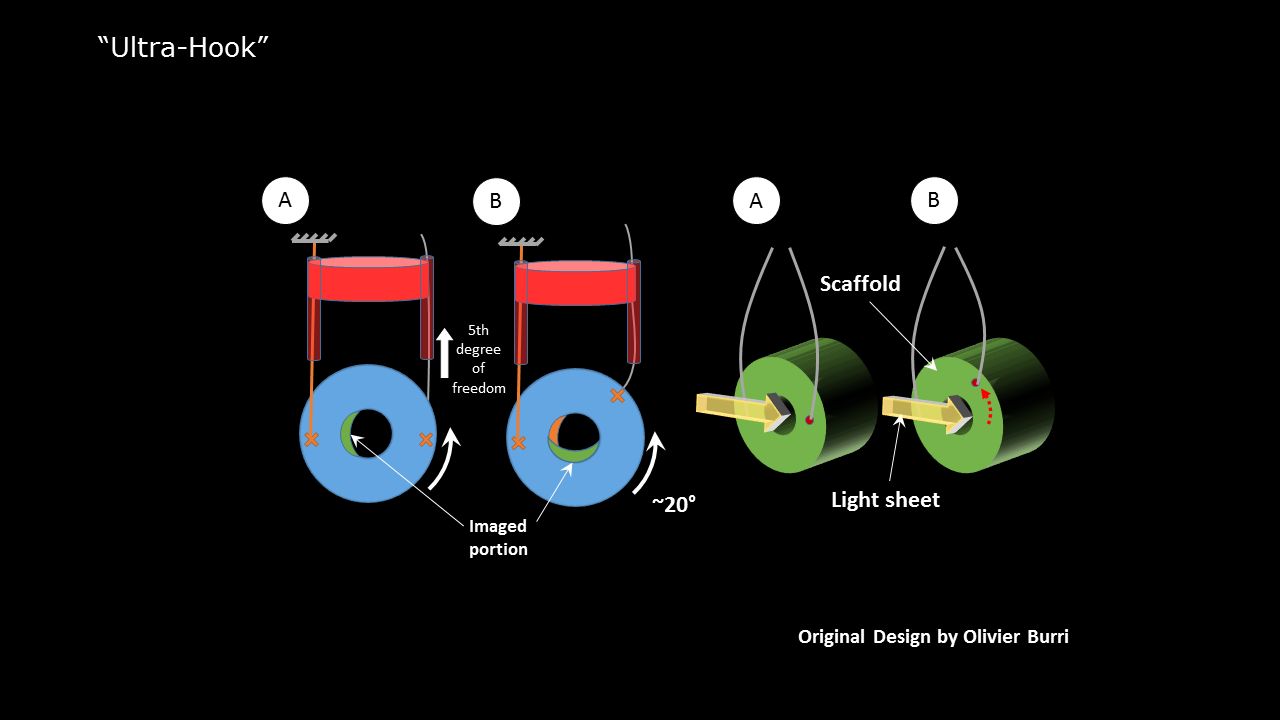
Unfortunately, due to the scaffold design and limitations of the objective lens, the conventional mounting technique using FEP-tubes or agarose was not applicable. Therefore the sample has been mounted on a double-hook and imaged from multiple views. The obtained multi view images were then reconstructed in Fiji.
Due to the large amount of data (approx. 100GB for 6 views) and the fact that the scaffolds do not contain beads, reconstruction of the multi view images in Fiji is complicated and still in progress!
Sketch of the mounting technique to image scaffolds in a Zeiss Lightsheet.Z1. Click to enlarge image.
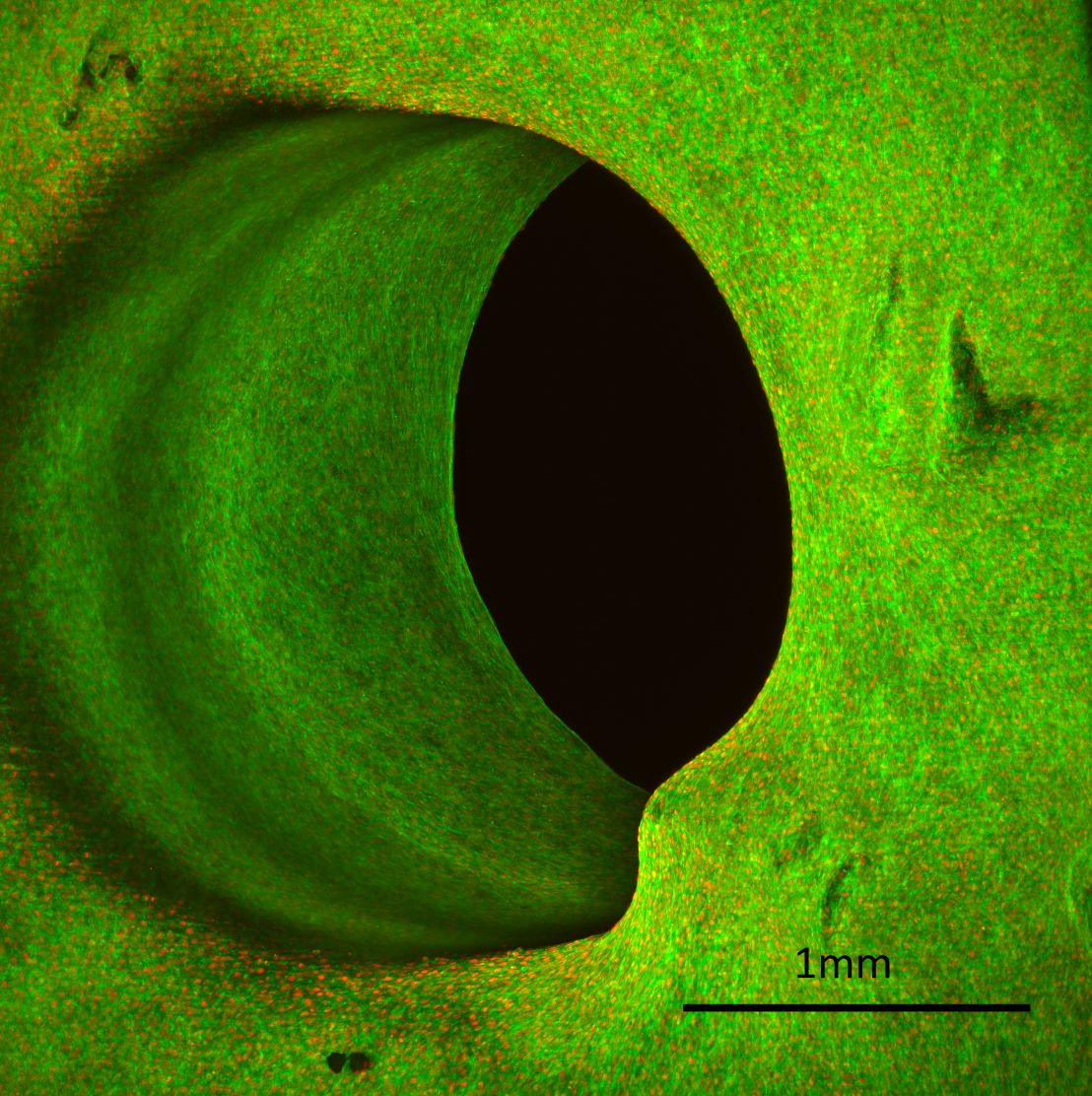
Tissue grown in a circular pore. The grey scale image shows the actin signal throughout the scaffold. The pore size is 2mm. Click to enlarge image.
Maximum projection of tissue grown in a circular pore obtained with Zeiss Lightsheet.Z1 and a 5x objective lens. One can clearly see cell-nuclei and actin-bundles. Actin fibres are depicted in green and cell-nuclei in red. The pore size is 2mm. Click to enlarge image.
3D reconstruction of multi-view images obtained with a Zeiss Lightsheet.Z1 Microscope. The 3D image has been reconstructed in Fiji. Cell-nuclei are depicted in blue and actin in magenta. Note that the presented 3D reconstruction of the multi-view images is still in progress.