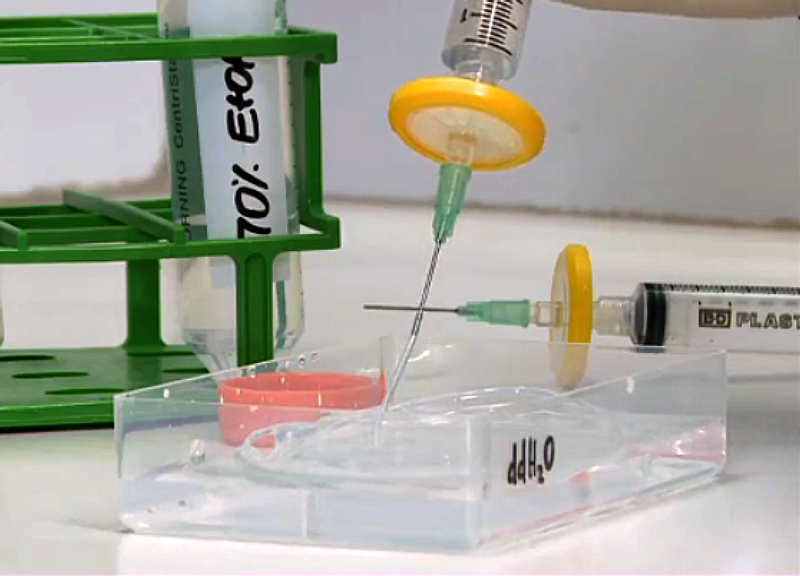
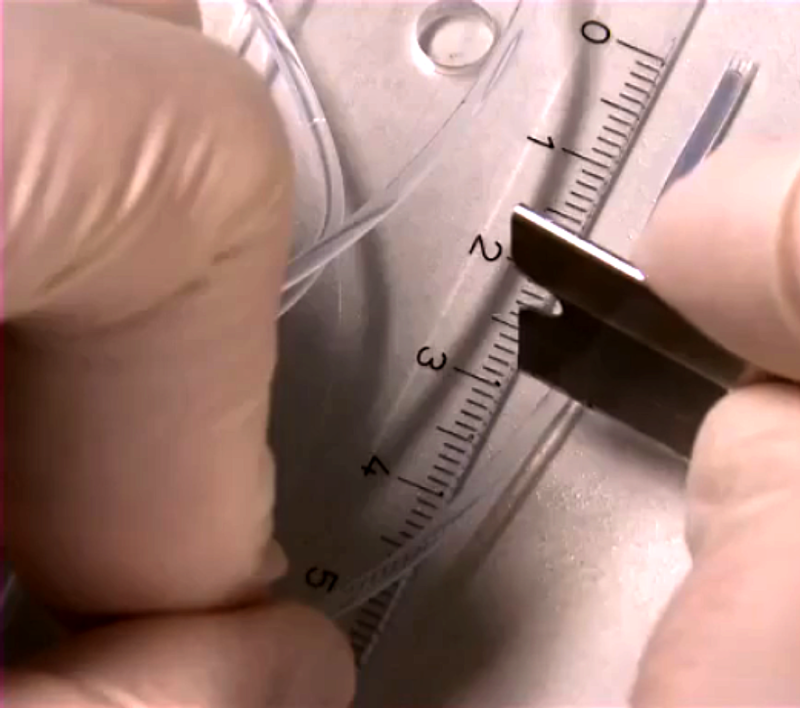
Zebrafish Embryo Sample Preparation
Zebrafish embryos should be mounted according to the imaging needs, e.g.:
- for short-term imaging: in 1.5% agarose inside a glass capillary, pushed out for imaging (the classic approach), w/ optional FEP tube support
- for long-term imaging: in 0.1% agarose inside a polymer (FEP) tube
- for long-term cardiac imaging: in 3% methylcellulose inside a polymer (FEP) tube
See the links to publications dealing with sample mounting for light sheet microscopy at the bottom of this page.

Material
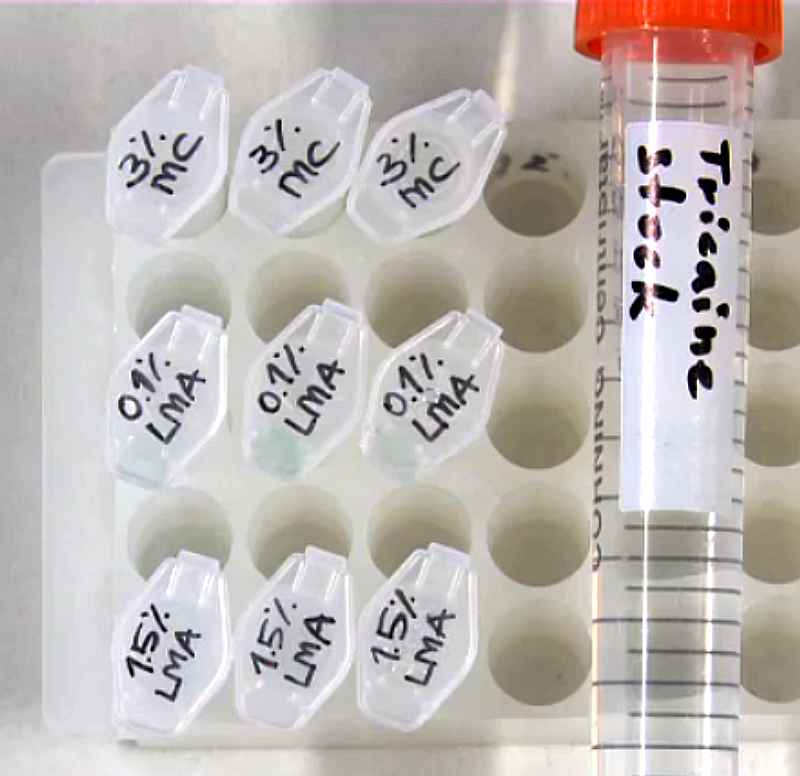
List
| product | external link | short-term imaging I | short-term imaging II | long-term imaging | long-term cardiac imaging | |
| E3 fish medium | - | Zebrafish: a practical approach (Nüsslein-Volhard and Dahm, 2002) | o | o | o | o |
| Tricaine/MS-222/TS 222 | Aldrich/Sigma E10521 | Sigma-Aldrich | o | o | o | o |
| agarose, low gelling temperature | BioReagent/Sigma A9414 | Sigma-Aldrich | o | o | o | o |
| methyl cellulose | Sigma M0387 | Sigma-Aldrich | - | - | - | o |
| glass capillaries | 1) Brand 701904 glass capillaries 20 µl black (accessory/spare part for Transferpettor) or 2) Wheaton 851322 glass capillaries 25 µl, 1 mm diameter, 125 mm in length | 1)Brand 2)Wheaton | o | - | - | - |
| teflon-coated plungers | 1) Brand 701932 teflon-coated plungers 10µl/Dig.25µl (accessory/spare part for Transferpettor) or 2) Wheaton 851331 teflon-coated plungers | 1) Brand 2)Wheaton | o | - | - | - |
| 1 ml syringe | 1) B. Braun Omnifix F Solo 1 ml Syringe or 2) BD tuberculin syringe 309659, 1ml | 1) B. Braun 2)BD | - | o | o | o |
| hypodermic needle | B. Braun 100 Sterican, blunt | B. Braun | - | o | o | o |
| FEP tubes | Bola S1815-04, inner diameter 0.8 mm, outer diameter 1.6 mm | Bola | - | o | o | o |
Prepare agarose stock
- dissolve agarose powder in E3 in a glass bottle to a final concentration of 0.1/1.5%
- heat up the mixture in a microwave w/o boiling it
- distribute 1 ml aliquots in 1.5 ml reaction tubes
- keep the aliquots in the fridge
Prepare agarose for mounting
- melt aliquots of agarose in a heat-block at 70 deg Celsius
- vortex the aliquots
- keep them in a heat-block at 38 deg Celsius
Prepare agarose plates
- melt aliquots of agarose in a heat-block at 70 deg Celsius
- vortex the aliquots
- pour 2 aliquots of agarose into a 35 mm plastic dish
Prepare methylcellulose stock
Prepare methylcellulose for mounting
Clean & cut FEP tubes
- flush tubes with 1 M NaOH, use syringe with attached needle
- transfer flushed tubes to fresh Falcon with 0.5 M NaOH, use forceps
- put the Falcon in an ultrasonic bath for 10 min
- from now on, touch the tubes only with gloves and/or forceps!
- transfer the tubes from the Falcon into small basin with ddH2O and flush them with ddH2O
- flush the tubes with 70% EtOH
- transfer the tubes to a fresh Falcon with 70% EtOH
- put the Falcon in an ultrasonic bath for 10 min
- cut the tube to small pieces of appropriate length
- transfer the tubes to a fresh Falcon with ddH2O for storage
Prepare syringes w/ FEP tubes
- take a cleaned and cut FEP tube and carefully attach it to the needle
- do not touch the part of the tube that will later be used for imaging
Coat tubes w/ methyl cellulose
- slowly take up 3% methyl cellulose with a needle-attached FEP tube
- slowly push out the methyl cellulose and discard it
- immediately proceed with mounting
Prepare Zebrafish embryos
Short-term imaging I: the classic approach
The method of choice mounting Zebrafish embryos is very similar to the one for mounting Drosophila embryos. It is simple and straight-forward, but restricts the embryo from growing. This limits the usable time frame for imaging to about 1-2 hours from the mounting.
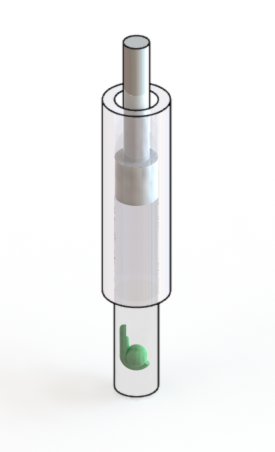
If required for multi-view registration, add fluorescent beads to the melted aliquots of agarose. Keep the agarose aliquots at 38 deg Celsius. Transfer a Zebrafish embryo of choice to the agarose and take it up with a glass capillary (inner diameter around 1 mm) and a plunger. Let the agarose solidify and keep the embryo in the capillary submerged in fish medium. For imaging, push out the part of the agarose which contains the embryo. If required, add Tricaine to the imaging chamber.
Short-term imaging II: FEP tube as additional support
- take up an embryo w/ a FEP tube which is attached to a needle and syringe
- wait a couple of minutes for the agarose to solidify and cut off the tube from the needle
- store the prepared sample in a 1.5 ml reaction tube filled with E3 fish medium
- quickly proceed w/ imaging
Long-term imaging
The limitations of mounting a zebrafish embryo in 1.5% agarose can be overcome by using a lower concentration of agarose plus a transparent solid support. To achieve unpersuaded image quality, the refractive index of the support has to match that of the medium. A good choice is FEP (fluorinated ethylene propylene). This polymer has a refractive index close to the one of water, and can be ordered as tube in various diameters right from the shelf. For imaging, the Zebrafish embryo is kept inside the cleaned and coated tube, embedded in 0.1% agarose with some Tricaine.
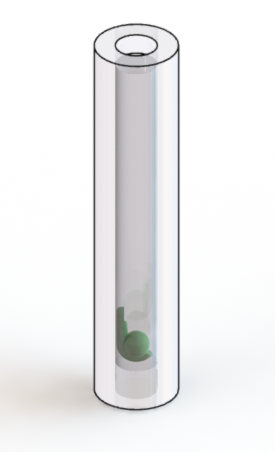
Long-term cardiac imaging

External links
- Multilayer Mounting for Long-term Light Sheet Microscopy of Zebrafish (external "JoVE" video article)
- Multilayer mounting enables long-term imaging of zebrafish development in a light sheet microscope (external "Development" article)